E.G. Shapiro, J.B. Eisengart, D. Whiteman, et al., Ability change across multiple domains in mucopolysaccharidosis (Sanfilippo syndrome) type IIIA, Molecular Genetics and Metabolism (2023),https://doi.org/10.1016/j.ymgme.2023.108110
Abstract
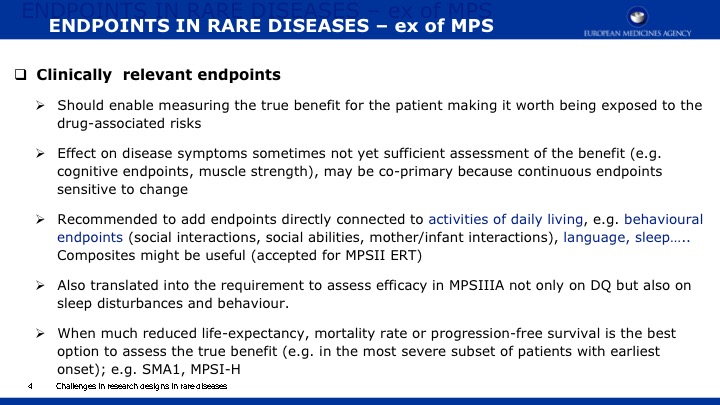
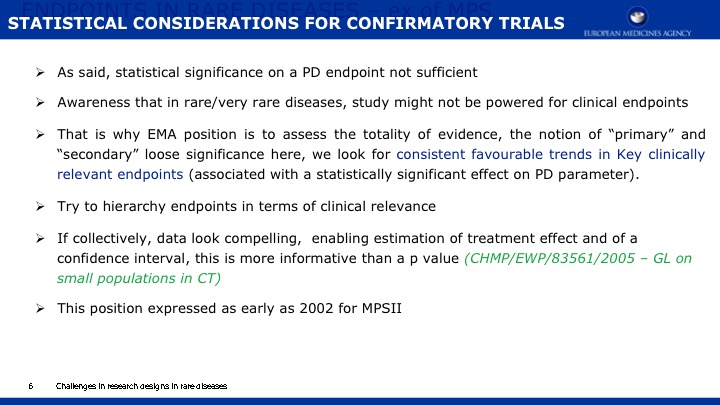
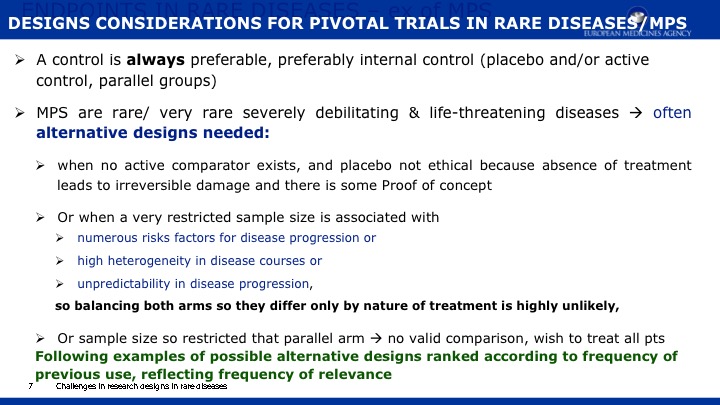
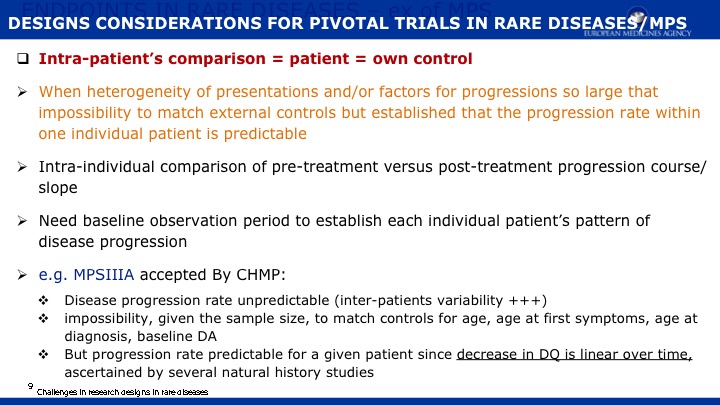
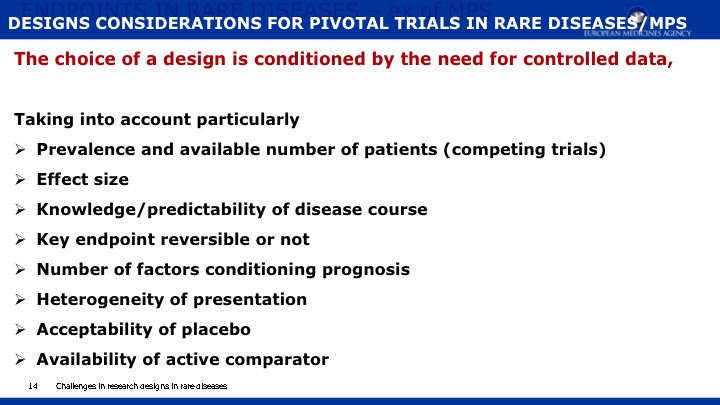
The objective of this paper is 1) to expand the scope of the domains previously published in a natural history study of Mucopolysaccharidosis IIIA (Sanfilippo syndrome type A) (MPS IIIA) and 2) to present evidence regarding the capacity of a new metric, Growth Scale Values (GSVs), in comparison with traditional metrics, to show changes in skills as assessed by the Bayley Scales of Infant Development -III (BSID-III) and the Vineland Adaptive Behavior Scales, Second Edition (VABS-II). We re-analyzed a cohort of 25 children, 20 with rapid progressing disease and 5 with slow progression, who had been followed over two years using the BSID-III, and the VABS-II. Previously findings were reported using age equivalent scores; now we are also presenting findings with GSVs. For the re-analysis, Language and Motor scores were added to the Cognitive scale on the BSID-III, and Domain- and Subdomain-level scores added to the Total VABS-II score (i.e., ABC Composite). We evaluated raw scores, age equivalent scores, and GSVs (and standard scores for the VABS-II only). Individual patient data can be found in the appendices to this publication.
Results indicate that 1) Cognition as measured by GSVs was the most sensitive to decline; 2) GSVs showed significant decline in the range of 4 to 6 years of age; 3) For children under 4 years of age, positive growth occurs on most scales and most metrics, with the exception of language which slows somewhat earlier; 4) Other than the Cognitive scale, Receptive Language on the BSID-III and Receptive Communication on the VABS-II showed the most sensitivity to change; 5) Gross Motor skills showed the least decline over time and appeared to lack sensitivity to MPS IIIA motor concerns; and 6) No evidence for sensitivity to change for any metric was found in time intervals less than one year.
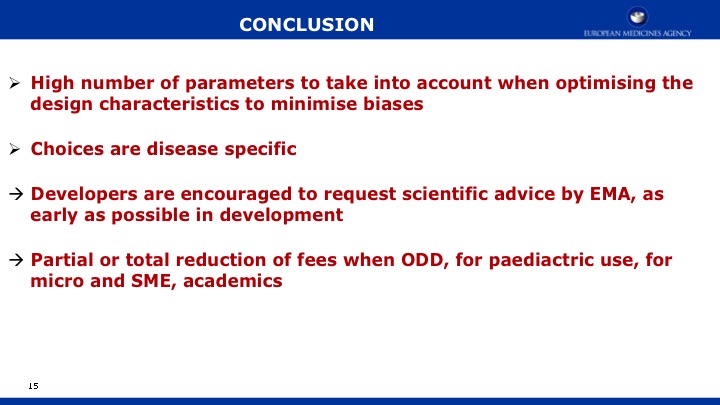
We conclude that GSVs are a precise measurement of change to detect decline in function, and they are a valuable method for future clinical trials in MPS IIIA. Evidence continues to support cognition as a primary endpoint. Additional work is needed to identify sensitive measures of meaningful endpoints to families.