This webinar was designed for clinical specialists, investigators, and pharmaceutical representatives who work in therapy development.
View the webinar
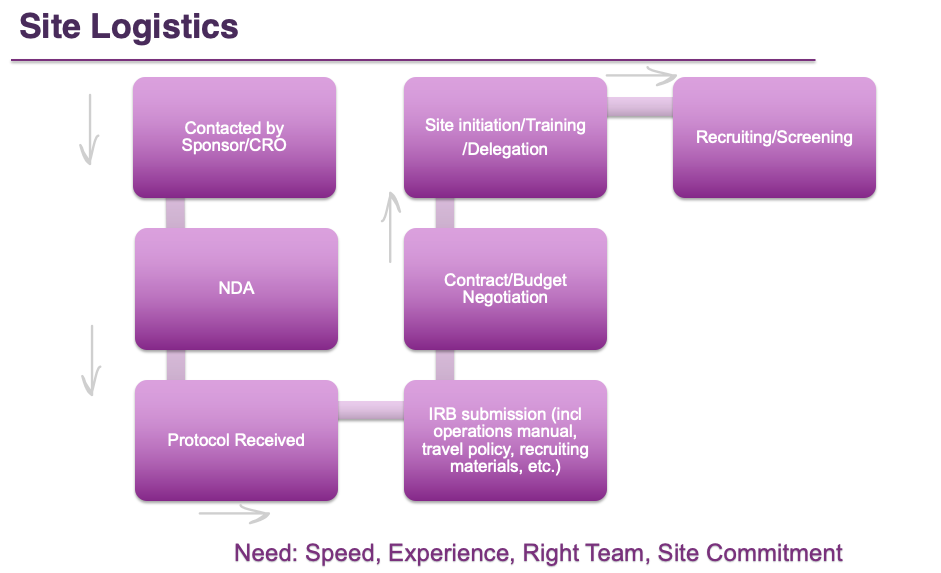
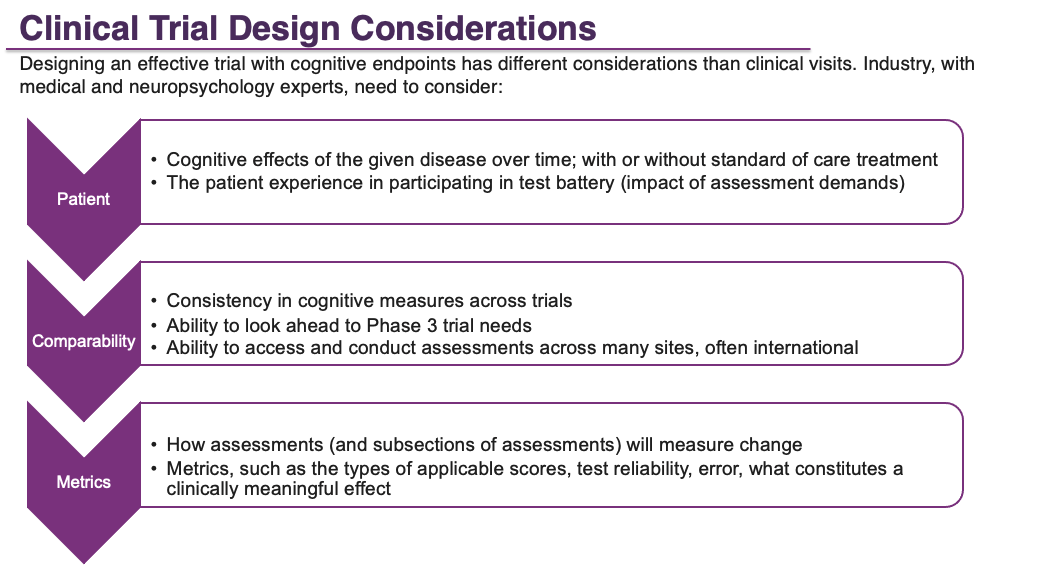
Rare disease drug development is made possible through strong industry partnerships with academic and medical experts. When diseases involve the central nervous system, trial design is even more complex. It requires partnering with experts who have specific disease experience, who understand measurements, and who are familiar with the complexities of clinical trials.
Elsa Shapiro, PhD and Paul Harmatz, MD, present an informative conversation on the unique role of the consulting psychologist, building partnerships within the greater trial ecosystem, and considerations for protocol development.
Paul Harmatz, MD: Dr. Harmatz is Professor in Residence at UCSF Benioff Children’s Hospital Oakland, California. He completed his Pediatric internship and residency training at Harbor-UCLA Medical Center. Following a research fellowship in Pediatric Gastroenterology and Nutrition at Massachusetts General Hospital, he remained in Boston until 1992 as faculty in Pediatrics at Harvard Medical School. During the last 15 years, Dr. Harmatz has participated in clinical trials with MPS I, MPS II, IVA, VI, and VII and has managed clinical care for MPS patients living in northern California.